Abstract
Introduction: Subsequent neoplasms (SN) represent a leading cause of non-relapse mortality among hematopoietic cell transplantation (HCT) survivors. Treatment advances and expanded access for older patients have resulted in a growing population of HCT survivors. Changes in the clinical approaches to HCT, including the introduction of reduced-intensity conditioning regimens, have changed the landscape of potential SN risk factors. The Center for International Blood and Marrow Transplant Research (CIBMTR) collects extensive patient, procedure, and outcome data from HCT centers in the United States and abroad and serves as a key resource for quantifying SN incidence and identifying risk factors. The completeness of SN and other late effects reported to the CIBMTR is uncertain because patients may not continue follow-up care, particularly long-term care, at HCT centers. The California Cancer Registry (CCR) is a statewide population-based cancer registry which is estimated to capture 99% of all cancer diagnoses occurring among state residents. We compared the distribution and cumulative incidence of solid SNs within a cohort of individuals who underwent HCT for hematologic malignancy reported to the CIBMTR and linked to the California Cancer Registry (CCR).
Methods: The study population included 18,450 California residents from the CIBMTR (8,232 allogeneic and 10,218 autologous HCT recipients) who were diagnosed with a hematologic malignancy between 1991-2016, linked to the CCR, and had known dates of first HCT, death (if deceased), or last known date alive. Patients were followed from the date of first HCT until the earliest of a second HCT, death, or date last known alive according to either source. ICD-O-3 histology and topography codes were used to identify and classify solid tumors in the CCR according to 10 broad types available from CIBMTR (see Table 1 below). We excluded non-melanoma skin cancers, as cutaneous basal and squamous cell carcinomas are not reportable to the CCR. We matched reports of solid SNs from the two sources according to type and diagnosis date. Cumulative incidence of developing a solid SN, accounting for the competing risk of death, was estimated from CCR and CIBMTR separately and using both data sources.
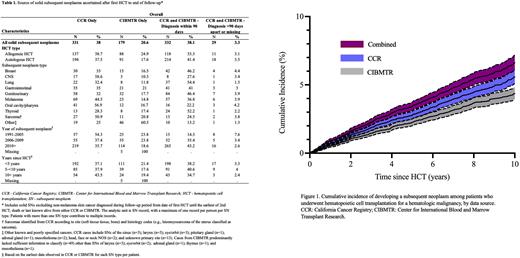
Results: During a median time since HCT of 2.4 years (range: 0.003 to 27.4 years), CIBMTR and CCR reported solid SNs among 518 and 641 HCT survivors, respectively. In CCR, 7.5% of patients with an SN (48/641) had more than one type of SN; in CIBMTR the corresponding estimate was 4.1% (21/518). Among 775 unique patients with at least one solid SN from either source, 384 (49.6%) had an SN reported from both sources, 257 (33.2%) from CCR only and 134 (17.3%) from CIBMTR only. As patients could have multiple SN types, the combined dataset included 871 solid SNs, of which 38.0% were found in CCR only, 20.6% in CIBMTR only, 38.1% were found in both sources and had diagnosis dates within 90 days, and the remaining 3.3% were found in both sources but had discrepant dates (Table 1). The proportion of SNs found in both sources varied by HCT type, SN type, calendar year of SN diagnosis, and time since first HCT. Specifically, the proportion identified in both sources was higher after autologous than allogeneic HCT. By SN type, >50% of SNs of the lung, thyroid, GU system, and breast were found in both sources; a majority of sarcomas and SNs of the oral cavity/pharynx and CNS tumors were found in CCR only; and other/poorly specified SN types were more likely to be found in CIBMTR only, driven by poorly specified SNs. The proportion of cases found in both sources and concordant on diagnosis date was lower for SNs diagnosed in earlier calendar years (<2010) and those occurring >10 years since HCT. The cumulative incidence of developing a solid SN at 10 years following a first HCT was 4.4% (95% cumulative incidence [CI] 3.9%-4.8%) based on CIBMTR information, 5.6% (95% CI] 5.1% to 6.1%) based on CCR information, and 6.6% (95% CI 6.1%-7.2%) based on both sources combined (Figure 1).
Conclusion: In this linkage of CIBMTR data with population-based cancer registry data, 38.0% of solid SNs were found in CCR only, 20.6% in CIBMTR only, and 41.4% in both sources. These analyses suggest that linkage of these high-quality data sources can offer a more complete assessment of the burden of solid SNs among HCT survivors.
Disclosures
Meyer:Genzyme: Other: Study. Wun:GBT, Inc.: Membership on an entity's Board of Directors or advisory committees. Auletta:AscellaHealth: Membership on an entity's Board of Directors or advisory committees. Muffly:Jasper: Research Funding; Adaptive: Honoraria, Research Funding; BMS: Research Funding; Adaptive: Honoraria; UpToDate: Consultancy, Honoraria; Novartis: Research Funding; Astellas: Consultancy, Research Funding; CTI Biopharma: Consultancy; Medexus: Consultancy; Kite: Consultancy, Research Funding; Pfizer: Consultancy; Amgen: Consultancy. Keegan:GRAIL: Other: Cancer Survivorship Advisory Board Meeting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal